Entropy and Free Energy in Structural Biology: Unlocking the Secrets of Protein Folding and Molecular Machines

In the intricate world of biology, the understanding of molecular interactions and the driving forces behind protein folding and molecular machine function is a fundamental aspect of understanding life itself.
The concepts of entropy and free energy play a crucial role in this fascinating field, providing a lens through which we can comprehend the inner workings of these complex biological systems. This article will delve into the concepts of entropy and free energy, exploring their significance in structural biology and highlighting their applications in understanding protein folding, molecular machines, and the dynamics of biological processes.
5 out of 5
| Language | : | English |
| File size | : | 19541 KB |
| X-Ray for textbooks | : | Enabled |
| Print length | : | 396 pages |
In this article, we will explore the physical principles underlying protein folding, examining how the interplay of entropy and free energy guides the formation of intricate protein structures. We will also investigate the role of entropy in molecular machines, shedding light on how these molecular entities harness energy to perform their biological functions.
Furthermore, we will uncover how entropy and free energy can be manipulated to influence protein folding and molecular machine function, opening avenues for therapeutic interventions and biotechnology applications.
Entropy in Structural Biology
Entropy, a fundamental concept in thermodynamics, measures the degree of disFree Download or randomness within a system. In the context of structural biology, entropy plays a pivotal role in driving protein folding and guiding the assembly of molecular machines.
During protein folding, the polypeptide chain undergoes a remarkable transformation, transitioning from a disFree Downloaded state to a highly Free Downloaded and functional conformation. This process is largely influenced by the interplay between entropy and free energy.
As the polypeptide chain folds, it loses degrees of freedom, resulting in a decrease in conformational entropy. However, this reduction in entropy is compensated by an increase in enthalpic interactions, such as the formation of hydrogen bonds, hydrophobic interactions, and van der Waals forces. These enthalpic interactions stabilize the folded structure, lowering the free energy of the system.
The balance between conformational entropy and enthalpic interactions determines the stability and dynamics of protein structures. Proteins typically fold into their functional conformations when the free energy of the folded state is minimized. This delicate balance ensures that proteins can adopt their specific structures while maintaining the flexibility required for their biological functions.
Free Energy in Structural Biology
Free energy, a thermodynamic potential, represents the amount of energy available to do useful work within a system. In structural biology, free energy plays a central role in driving protein folding and determining the equilibrium distribution of molecular conformations.
The free energy of a protein is influenced by various factors, including its enthalpy, entropy, and the surrounding environment. Under physiological conditions, proteins fold into conformations that minimize their free energy. This process is governed by the principle of least free energy, which states that a system will tend to adopt the state with the lowest free energy.
The free energy landscape of a protein, a graphical representation of the free energy as a function of its conformational space, provides insights into the folding pathways and stability of the protein. By analyzing the free energy landscape, researchers can identify the transition states and intermediates involved in protein folding, shedding light on the dynamic nature of these processes.
Furthermore, free energy calculations are essential for predicting protein-protein interactions, ligand binding, and the thermodynamics of molecular machines. By harnessing computational methods and experimental techniques, scientists can determine the free energy changes associated with these interactions, providing valuable information for drug design and biotechnology applications.
Entropy and Free Energy in Molecular Machines
Molecular machines, intricate assemblies of proteins and nucleic acids, are responsible for carrying out essential cellular functions, ranging from energy transduction to DNA replication. These remarkable molecular entities harness energy from their surroundings to perform their biological tasks.
Entropy plays a crucial role in the operation of molecular machines. By coupling their actions to entropy-driven processes, molecular machines can efficiently perform their functions while dissipating heat. For instance, the ATPase molecular motor utilizes the hydrolysis of ATP to power its movement along microtubule tracks. The entropy increase associated with ATP hydrolysis provides the driving force for the motor's motion.
Free energy is also a fundamental aspect of molecular machine function. The free energy difference between the bound and unbound states of ligands and substrates determines the affinity and specificity of these interactions. By modulating the free energy landscape of molecular machines, researchers can manipulate their activity and specificity, opening avenues for therapeutic interventions and the development of novel molecular tools.
Applications in Biotechnology and Drug Design
The understanding of entropy and free energy in structural biology has significant implications in biotechnology and drug design. By manipulating these thermodynamic parameters, scientists can engineer proteins with enhanced stability, specificity, and activity.
In the field of biotechnology, entropy and free energy can be harnessed to improve the production of therapeutic proteins. By optimizing the folding pathways and free energy landscapes of these proteins, researchers can increase their yield and reduce the formation of misfolded aggregates.
Drug design also benefits from the knowledge of entropy and free energy. By targeting specific protein-ligand interactions, scientists can design drugs that modulate the free energy of binding, thereby enhancing their potency and specificity. This approach has led to the development of novel drugs for various diseases, including cancer and infectious diseases.
Furthermore, the principles of entropy and free energy guide the design of synthetic molecular machines. By mimicking the entropy-driven mechanisms employed by natural molecular machines, scientists can create artificial systems capable of performing complex tasks, ranging from drug delivery to environmental sensing.
Entropy and free energy are fundamental concepts that permeate the field of structural biology, providing a powerful lens through which we can comprehend the intricate workings of proteins and molecular machines. By understanding the interplay between these thermodynamic parameters, scientists can unravel the secrets of protein folding, decipher the mechanisms of molecular machines, and harness these principles for biotechnology and drug design applications.
As we continue to probe the depths of structural biology, the concepts of entropy and free energy will undoubtedly remain central to our understanding of the molecular basis of life. Through continued research and technological advancements, we can unlock even greater insights into the dynamic and fascinating world of biological systems.
5 out of 5
| Language | : | English |
| File size | : | 19541 KB |
| X-Ray for textbooks | : | Enabled |
| Print length | : | 396 pages |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Taro Gomi
Taro Gomi Anni Gethin
Anni Gethin Andre Gide
Andre Gide Wes Crenshaw
Wes Crenshaw Dr Daryl Gioffre
Dr Daryl Gioffre Carol Crowe Carraco
Carol Crowe Carraco Thomas Harding
Thomas Harding Yogesh Patel
Yogesh Patel Marjorie Howe
Marjorie Howe J W Rinzler
J W Rinzler Paul Lowe
Paul Lowe Andre Parker
Andre Parker Gregor Craigie
Gregor Craigie Jacob Glass
Jacob Glass Mark Jones
Mark Jones Gita Joshi
Gita Joshi Lloyd Kahn
Lloyd Kahn Machi De Waard
Machi De Waard Chip Jacobs
Chip Jacobs Terrell Clements
Terrell Clements
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Charles DickensHow a Few Brave Americans Risked All to Save Our Vietnamese Allies at the End...
Charles DickensHow a Few Brave Americans Risked All to Save Our Vietnamese Allies at the End... Michael SimmonsFollow ·7.6k
Michael SimmonsFollow ·7.6k Ervin BellFollow ·4.4k
Ervin BellFollow ·4.4k Carter HayesFollow ·18.4k
Carter HayesFollow ·18.4k J.D. SalingerFollow ·10.5k
J.D. SalingerFollow ·10.5k Henry GreenFollow ·12.8k
Henry GreenFollow ·12.8k Corey HayesFollow ·18.3k
Corey HayesFollow ·18.3k Kendall WardFollow ·16.4k
Kendall WardFollow ·16.4k Braeden HayesFollow ·13.1k
Braeden HayesFollow ·13.1k

 Henry Green
Henry GreenCorrosion and Its Consequences for Reinforced Concrete...
Corrosion is a major threat to reinforced...

 James Gray
James GrayDiscover the Enigmatic World of Pascin in "Pascin Mega...
Immerse Yourself in the...

 George R.R. Martin
George R.R. MartinUnlocking the Power of Nature: Delve into the Bioactive...
In a world increasingly...

 Julian Powell
Julian PowellMaster the Art of Apple Watch App Development: A...
Unlock the Potential of Apple Watch Apps In...
 Jaylen Mitchell
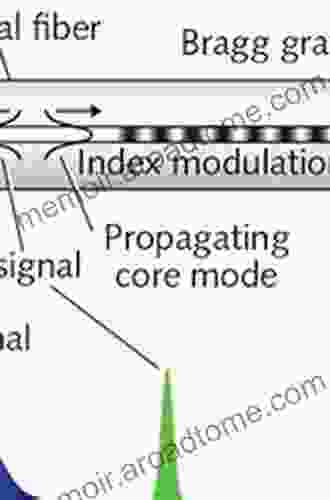
Jaylen MitchellPlastic Optical Fiber Sensors: A Comprehensive Guide to...
In the rapidly evolving landscape of...

 Truman Capote
Truman CapoteUnlock the Secrets of Language Creation: Dive into...
The realm of computer science...
5 out of 5
| Language | : | English |
| File size | : | 19541 KB |
| X-Ray for textbooks | : | Enabled |
| Print length | : | 396 pages |